LM-Abs™ (Targeted Antibody Discovery Platform)
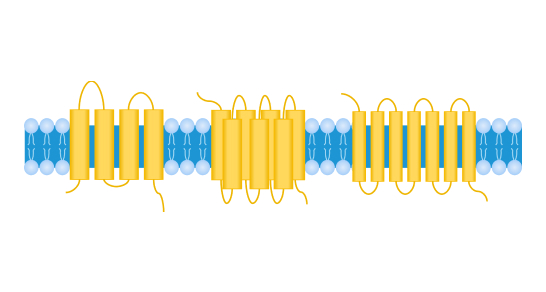
Multi-transmembrane protein
Transmembrane proteins (TPs) are a class of protein molecules that possess multiple hydrophobic transmembrane domains and span the intra- and extracellular environment by intercalating in the phospholipid bilayer of the cell membrane. According to the structural composition of the transmembrane segments, transmembrane proteins can be divided into different classes such as single span (bitopic) or multi-span (polytopic, or multi-transmembrane). Typical representatives of multi-transmembrane proteins include four-transmembrane protein Claudin 18.2, five-transmembrane protein CD133, and seven-transmembrane proteins GPCR5D, CCR8. Based on their functions, transmembrane proteins can be divided into G protein-coupled receptors (GPCRs), ion channels, transporters, and other families. Transmembrane proteins play key roles in human physiological processes such as molecular transport, signal transduction, and energy metabolism, and are involved in the regulation of various cellular structural and functional changes, thus becoming important targets for drug discovery.
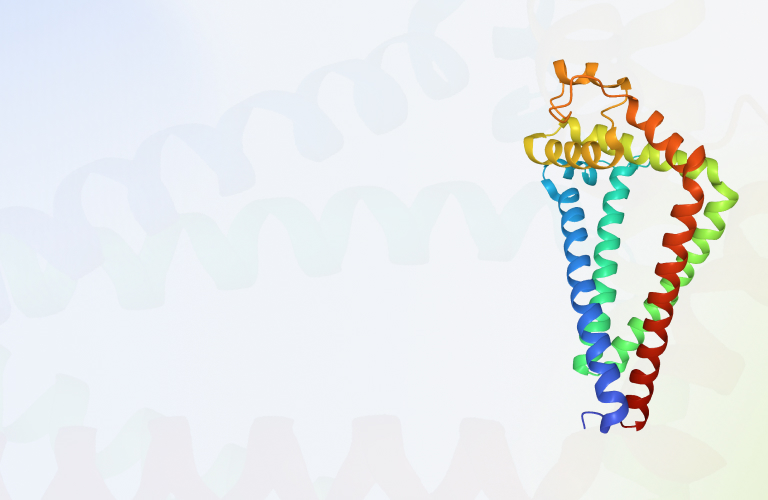
G protein coupled receptors
G protein coupled receptors (GPCRs) are the largest superfamily of membrane protein receptors in the human body and are widely distributed in various tissues and organs. GPCRs are classified as seven transmembrane proteins since their transmembrane domain contain seven a-helices.
It is estimated that about 800 member proteins are GPCRs, covering about 1/3 of the existing drugs targets on the market. With the progress of Immuno-oncology research, the role of G protein-coupled receptors in tumor immunosuppression and immune escape mechanisms has also become a research hotspot.
Currently, there are many GPCRs that are difficult to targeted by small molecules or peptides. The high specificity, selectivity and flexible modification of monoclonal antibodies make them ideal drugs to target GPCRs. However, due to the complex structure of multiple transmembrane proteins, their hydrophobic and lipophilic transmembrane regions need to rely on a phospholipid bilayer to maintain the correct folded structure. In addition, their expression level in host cells could also be extremely low. Therefore, it is difficult to prepare multiple transmembrane proteins with correct conformation and biological activity. Coupled with the complexity of immunization and antibody screening, there are many challenges remaining in the development and preparation of antibodies against G protein-coupled receptors.